
- Self-Study Guided Program o Notes o Tests o Videos o Action Plan

Salient Achievements 2020-2021
The Department of Biotechnology has made tremendous efforts in promoting Bioscience research, translational education and entrepreneurship. The Department has laid emphasis on the generation of biotech products, processes and technologies for enhanced efficiency, productivity and cost-effectiveness in the areas of agriculture, food and nutritional security; affordable health care and wellness; environmental safety; clean energy and biofuel, bio- manufacturing etc. Skill development programmes have been developed in close coordination with State Governments. The Department has contributed through its various programmes to the National Missions launched by the Prime Minister- Swasth Bharat, Swatch Bharat, Start-up India, Make in India and Digital India. The Department has also risen to the challenge for mitigation of COVID Pandemic by supporting vaccines, therapeutics, diagnostics, genomics, bioreporsitories and a National Platform (NBRIC) for achievement of Atmanirbharta.
Snap shot of achievements: The coordinated efforts of all the stakeholders resulted in supporting more than1642 projects, 2731Scientists and 5145 project fellows, more than 1 Lakh students were trained under Star college scheme, 40 Ramalingaswami Re-entry fellows secured regular appointment, 20,000 rural, SC,ST and Women beneficiaries were supported through Societal programme and more than 25,000 farmers supported thorough Biotech KISAN scheme.
UNATI Atal Jai Anusandhan Mission Programmes
UNATI Atal Jai Anusandhan Mission has been implemented by DBT with a major focus on improved agriculture, affordable healthcare, clean energy and cutting-edge frontier science as per details given below:
Ind-CEPI MISSION is an India centric collaborative mission of DBT aligned to the global initiatives of CEPI (Coalition of Epidemic Preparedness Innovations). DBT is supporting the implementation of the Ind-CEPIs mission “Epidemic preparedness through rapid vaccine development: Support of Indian vaccine development is aligned with the global initiative of the Coalition for Epidemic Preparedness Innovations (CEPI)”, at BIRAC, PSU of DBT.
Ind-CEPI Mission initiated the eCourse Series entitled “Strengthening Clinical Trial Research Capacity in Neighbouring Countries” primarily aimed towards skill development, capacity building and regional networking and coordination. A total of 4-Program 10 sessions series were conducted via online platform with total engagement of more than 750 participants from neighbouring countries like Afghanistan, Bangladesh, Bhutan, Maldives, Mauritius, Nepal and Srilanka.

DBTs response to COVID
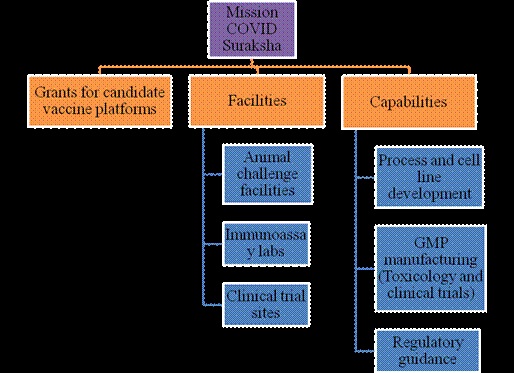
Mission COVID Suraksha was announced with a provision of Rs. 900 Cr. to DBT for supporting development of a comprehensive ecosystem for enabling the development of a safe, efficacious and affordable vaccine for COVID-19. The milestone is to accelerate the development of at least 5-6 vaccine candidates and ensure that some of these are brought closer to licensure for consideration of regulatory authorities and for introduction in public health systems.
Highlights of major activities include:
COVID Vaccines:
MEA accorded 'in principle' approval for financial support for phase 3 trial of COVAXIN in Bangladesh by Bharat Biotech International ltd. (BBIL). Signing of a tripartite HoA with BBIL and ICMR, to finalize the modalities and issues related to the conduct of a COVAXIN based Phase III Efficacy trial in Bangladesh is under consideration. The revised budgetary requirement provided by BBIL has been shared with MEA. The semi-executed tripartite Heads of Agreement was shared with ICMR, to finalize the modalities and issues related to the conduct of a COVAXIN based Phase III Efficacy trial in Bangladesh is under consideration. The proposal for the conduct of clinical trials of indigenously developed inactivated vaccine (COVAXIN) by Bharat Biotech International Ltd.(BBIL), in Bangladesh, is under consideration by the Ministry of External Affairs (MEA). DBT-BIRAC is facilitating the same.
· CDSCO had undertaken an inspection of the laboratory infrastructure and the facilities, at NCCS, Pune and NIAB, Hyderabad, for assessing their functioning as Central Drug Laboratories for COVID-19 vaccine testing. A detailed project report is being prepared for consideration of MoH&FW for expansion of the facilities.
· A discussion of Secretary, DBT with Officials from Myanmar was held on 21st December, 2020 to explore opportunities for association with DBT and procurement and deployment of Indian COVID-19 vaccines in Myanmar.
· A discussion was held with the leadership of CEPI on 21st December 2020 to discuss the potential contribution of CEPI to the clinical development of the RBD based protein subunit vaccine candidate for COVID-19, which was positively reviewed in the third call for proposals given by CEPI for COVID-19 vaccine candidate development.
COVID Therapeutics:
DBT-AYUSH partnership
DCGI approved First Phytopharma drug
Immunoglobulin based therapeutics
Clinical trial for equine immunoglobulin therapy to start shortly
DBT-RCB has set an in vitro cell culture-based assay to test the antiviral activity of potential molecules against SARS-CoV2. Services have been widely utilized by the academia and industry. Cytotoxicity Testing and anti-viral testing was done on 679 and 313 samples respectively
COVID Diagnostics:
Covid Genomics:
Augmentation of self reliance and support to Start-ups:

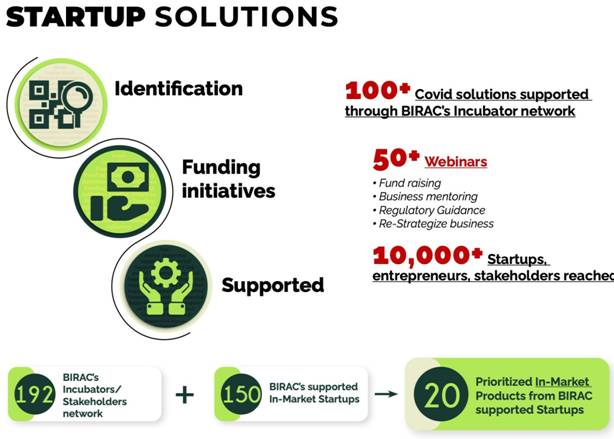
COVID Start-up solutions:
COVID Biorepositories:
Sectoral Achievements:
Health:
Product Development
Shared Facilities
Skill Development
Technology Transfer
Vaccines
11 vaccine candidates for Flu, Cholera, Dengue, Pneumonia, and COVID, under different stages of development are being supported
National Facilities
Creation of GLP, GMP, GCLP facilities besides cell line repositories and facilities for medical device testing and prototyping. Establishment of 19 national facilities are ongoing, out of which 04 are already operational.
Trainings & Workshops
1406 candidates have been trained in the areas of clinical research, regulatory compliances, technology transfer, biopharmaceuticals and medical devices. 37.12% of the participants were female.
Tech Transfer Offices (TTO)
Five TTO’s have been established with a view to strengthen the technology transfer capacity of the country.
Biotherapeutic mAbs
About 12 mAbs which are not presently existing in Indian market and 03 clones for diseases like cancer, diabetes, psoriatic arthritis, wet macular degeneration and lung infections caused by RSV are being supported. Additionally, one antibody-drug conjugate (ADC), one novel biologic, one recombinant monoclonal and one bio-better are also being supported.
Translational Research Consortia (TRC)
The Mission has supported two consortia
(i.) TRC for Dengue: 3 clinical sites and 5 premier institutes across the country led by ICGEB, Delhi.
(ii.) TRC for Chikungunya (CHKV): 4 hospitals and 3 premier research institutes across the county, led by Manipal Academy of Higher Education.
Device & Diagnostics
Currently 17 devices and 13 diagnostics are being supported. Support is being given for development of products in the areas of hospital-use equipment, diagnostic imaging, implants, wound-care products etc. Many diagnostic devices and reagents used for diagnostic kits are also supported including Molecular diagnostics, ELISA, LFT, sample transport reagents etc.
Clinical Trial Networks (CTN)
CTN for hospital-based trials in patients for testing biologicals in different specialties of oncology, diabetology, rheumatology and ophthalmology. Currently, 05 consortia including 36 hospitals are being supported. Strengthening the capacity of vaccine clinical trials, in already existing Demographic Surveillance sites and establishing new sites is another major focus area. Ten (10) Field Sites have been prepared for the same.
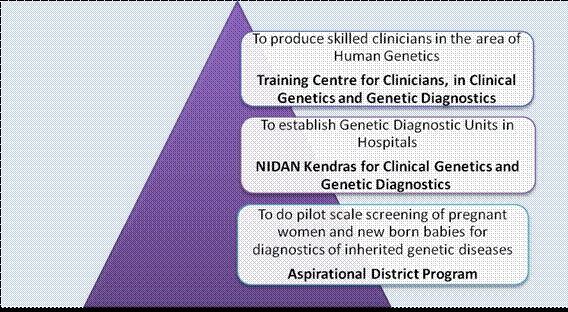

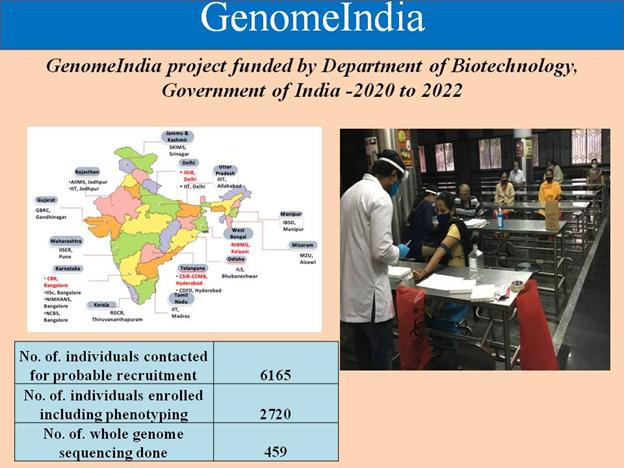

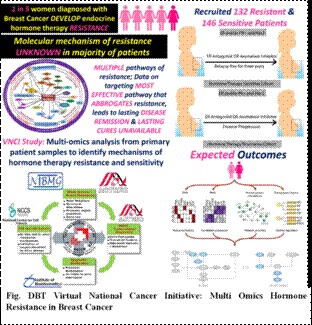
Agriculture and Allied Areas:
Agriculture Biotechnology

A few crop wise major achievements are placed below:
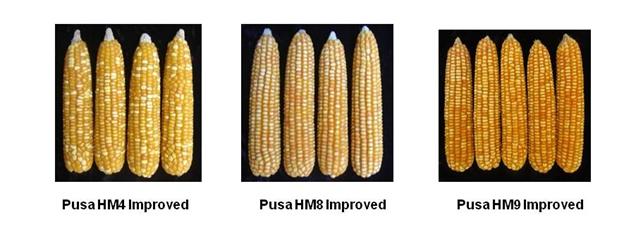
Animal Biotechnology:
Process/Product/ Technology Developed:
Process/Product/Technology Transferred to User Agency/ Industry/ Stakeholders:
Process/Product/Technology Commercialized:
Aquaculture:
Energy, Bioresources, Environment and Forest Biotechnology:
Knowledge Generation:

DBT-HRD Salient Achievements 2020-21:
DBT-Skill Vigyan Programme:
DBT Star College Scheme
Infrastructure
· Initiation of 3rd Phase of the “Access to Structural Biology Facilities at ESRF, France”: The European Synchrotron Radiation Facility (ESRF), located in Grenoble, France, is the world's most intense X-ray source and a centre of excellence for fundamental and innovation-driven research in condensed and living matter science. More than 300 Ph.D students and 1.52 lakhs researchers have utilized the services till date and has led to publication of more than 500 high impact research articles.
Conference, Travel, Exhibition and Popular Lectures (CTEP)
Societal Programme
Biotech-KISAN has created a platform in each of 15 agro-climatic zones of the country, which aims to connect farmers and scientists to promote Agriculture Innovation and take the new interventions to the farmers and farms. Biotech-KISAN Hubs have been established at pilot-scale level in all agro-climatic zones. The programme has now been scaled up and expanded its activities covering 105 Aspirational Districts in the country. The programme will lead to link the farmers with the latest scientific innovations with a view to enhance agricultural production and increasing farmers’ income. The programme has introduced 200 interventions for the benefit of farming community and more than 25000 farmers have been benefitted so far.
International Cooperation:
NER Program
Personals Trained in NER
Publications
Patents filed/Granted
Technologies transferred
Bioformulations developed at CoE AAU Jorhat –NECAB include Talc based formulations (Bio-Time; Biozin-PTB; Bio-Sona; Biollium, Bio-Meta; Bio-Zium; Bio-veer; Biogreen; Biogreen-L) and Organic based (Biofor-Pf). MoU was signed for transferring Bio-input (Biopesticide production technology) Technology to the three companies: VRS Agritech, Guwahati, School of Livelihood & Rural Development (SLRD), Shillong, M/s Orgaman R&D Division, Jorhat.
Entrepreneurship Development
Biotechnology Industry Research Assistant Council (BIRAC)

`
DBT - Autonomous Institutions
16 Autonomous Institutions are functioning under the aegis of the Department of Biotechnology. These institutions are pursuing basic, discovery and translational research in line with the National missions in the areas of Agriculture Biotechnology, Animal Biotechnology and health, Medical Biotechnology, Clean energy and Bioresourse development, Secondary Agriculture etc. The institutions also have a mandate of human resource development and societal outreach. Some of the achievements during the year are given below:
DBT-National Institute of Immunology (DBT-NII) has developed Mycobacterium indicuspranii based leprosy vaccine and immunomodulator. This is being marketed by Cadila Pharmaceuticals, Ahmedabad as Immuvac. DBT-NII has published 85 research papers in reputed journals, was granted 5 patents and filed 11 patent applications. In order to protect intellectual property rights of the research endeavors, two trademarks, ASPAGNII and PANII have been filed for vaccine and biotechnological composition respectively. During this year, the Institute has also sealed a Technology Transfer Agreement with Vitane Biologicals.
DBT-Center of Innovative and Applied Bioprocessing (DBT-CIAB) has received the granted status of its 7 patents on various bioprocess based technologies/products developed by the institute. 5 new technologies were also developed in the areas of biomass valorization and bioprocess technology. R & D has established that low-methoxyl pectin stabilizes low-fat set yoghurt and improves their physicochemical properties, rheology, microstructure and sensory liking. Processes for removal of bitterness from kinnow pomace and kinnow pulp residue were also developed.
DBT-National Institute of Animal Biotechnology (DBT-NIAB), Hyderabad has developed a high-density SNP chip using NGS of 43 Indian breeds of cows. This chip is currently under validation using 50 samples of each breed to identify breed specific signatures. NIAB developed a kit for detection of subclinical and clinical mastitis and assessment of microbial quality of milk using magnetic nanoparticles, without the need of any high end equipment . The overall cost to perform one test is less than US $ 0.50/test.
DBT-Regional Centre for Biotechnology (DBT-RCB): RCB has been providing service to academia and industry for testing potential antivirals against SARS-CoV-2 in the cell culture model. So far, over 900 compounds or herbal preparations have been tested. More than 100 students are pursuing doctoral degree programs in Biotechnology, Bioinformatics, and Biostatistics in different RCB laboratories. 25 students are currently registered with RCB for integrated MSc-PhD degree program.
DBT-National Agri-Food Biotechnology Institute (DBT-NABI) has developed a wheat straw polysaccharide derived novel edible coating formulation to prevent the post-harvest shelf-life of perishable fruit crops such as Apple, Peach and Banana. In addition to that, antibody assisted graphene oxide coated gold nanoparticles for rapid bacterial detection and near infrared light enhanced antibacterial activity has been developed which detects food borne pathogens.
DBT- Rajiv Gandhi Centre for Biotechnology (DBT-RGCB): DBT- RGCB has established a nationwide investigator network for research on Head and Neck Cancer ( HNC) alongwith a HNC Bio-repository Utroside-B was identified as a potential anticancer agent against liver Cancer. This compound was licenced to Q-Biomed for further development in a joint initiative with RGCB and OMRF.
DBT-inStem: DBT- inStem was ranked in the top 50 among Indian institutions and 7th in the Life Sciences in India by Nature insex 2020 and has more than thirty publications to its credit since April 2020. InStem scientists have devised probes to enable understanding the many roles of cellular actin which is an important conserved protein from microorganisms to complex organisms and is an emerging drug-target in malaria, leishmaniasis amongst others. Under the aegis of the ADBS programme a recent study examined the consequences of adverse childhood experiences and published a study that has taken an impressive leap forward in modelling Autism – a major and growing public health challenge – in the culture dish. Together, these observations offer new potential therapeutic targets that can be assessed in pre-clinical platforms and eventually lead to more efficient translation to the clinic.
DBT- National Centre for Cell Science (DBT-NCCS): A GMP-Compliant National Repository for banking, safe deposit and supply of characterized mammalian cells for use in Bio-Pharma under BIRAC’s National Biopharma Mission was established in the institute. Promising proteins were identified that could serve as biomarkers and therapeutic targets for multiple myeloma, the second most prevalent blood cancer at the global level.
DBT- Institute of Bioresources and Sustainable Development (DBT-IBSD): Himalayan Bioresources Mission was initiated to connect the East and West, with major emphasis on the development of bio resources of Himalaya and their utilization for future development. In microbial resource programme, microbial repository deposits have increased to more than 72,000 cultures including bacteria, fungi, actinomycetes and yeasts.
DBT-Centre for DNA Fingerprinting and Diagnostics (DBT-CDFD) has provided a) High quality forensic DNA fingerprinting services in 33 cases to the various courts of law, law enforcement and investigative agencies of the country; b) Genetic diagnostic services, prenatal diagnosis, and clinical genetic counselling services to more than 500 people, c) Plant DNA fingerprinting services for 490 samples. 33 research papers have been published and 1 patent was filed.
DBT-National Institute of Plant Genome Research New Delhi: Through marker-assisted selection, large seed characteristic has been transferred into a commercially important desi chickpea variety, JG11 (ICCV 93954). The Improved JG1 variety has a 15-20% increase in yield over the parent. This variety is undergoing Advanced Varietal Trial-2 (AVT-2) at 16 different rainfed areas of India.
Translational Health Science and Technology Institute (THSTI), Faridabad established a dengue virus repository of 40 isolates that are being characterized for vaccine development, dengue structural E protein platform to support antibody-based therapeutic discovery and development and AG129 mouse breeding and colony to facilitate dengue research, Identified a novel HIV-1 Indian clade C native like trimer antigen that has demonstrated favourable immunogenicity in rabbit model, solved the crystal structure of vapB12 antitoxin and generated a hetero-octameric model of vapBC12 toxin-antitoxin complex etc. DBT-THSTI established the following national facilities to support vaccine development:
• The institute has published 28 papers, filed 8 patent applications, one copyright application, one trademark application,4 technology developed, 4 technologies transferred, and 1 technology has been commercialized during the year.
DBT- Institute of Life Sciences (DBT-ILS): The institute published 35 research publications during the year 2020. A novel target for regaining sensitivity towards Tamoxifen in case of breast cancer was identified. Human IRGM was shown as a master suppressor of interferon-signaling and targeting IRGM induces a broad antiviral response which can be further developed for anti-viral therapeutics. The Institute of Life Sciences has transferred a technology for production of curcumin sponge for wound healing for commercialization.
DBT-International Center for Genetic Engineering and Biotechnology (DBT-ICGEB): ICGEB scientists during the year were able to raise 4.92 million USD through competitive external project funding and also published 104 publications in peer-reviewed journals. Institute has filed one (1) national and three (3) international Patents and was granted one (1) Patent in 2020.
A link was established between Mtb infection, epigenetics and host immune response, which can be exploited to achieve therapeutic benefits. Using in silico and in vitro screening strategies, potent inhibitors of major cysteine proteases of Plasmodium falciparum, falcipain-2/3, were identified (Bioorg Med Chem. 2020. 28:115155.). Trehalose accumulation was achieved in transgenic rice plants which enabled these plants to tolerate multiple abiotic stresses such as salinity, drought and sodicity and show minimum yield penalty under stress conditions.
DBT- National Institute of Biomedical Genomics (DBT-NIBMG), Kalyani: The ICGC – India Project team of NIBMG participated in the Pan Cancer Analysis of Whole Genomes, a large scale international collaborative initiative of ICGC and TCGA which undertook integrative analysis of 2,658 whole-cancer genomes and their matching normal tissues across 38 tumour types from the Pan-Cancer Analysis of Whole Genomes (PCAWG) Consortium of the International Cancer Genome Consortium (ICGC) and The Cancer Genome Atlas (TCGA). The habit of using RAN/tobacco and GSTP1 AA-genotype together play a significant role in predisposition to oral cancer risk. progress. The institute has published 29 articles, has supported 30 PhD students and 17 iPhD students.
DBT-National Brain Research Institute (DBT-NBRC), Manesar: Japanese Encephalitis virus (JEV) infection was found to induce classical activation (M1) of microglia that drives the production of pro-inflammatory cytokines, while suppressing alternative activation (M2) that could serve to dampen the inflammatory response, thereby inducing inflammatory response, microglial activation, and neuronal apoptosis.Sleep loss, a pathological condition during aging and Alzheimer’s disease, led to impairment of translation at synapses associated with memory storage. DBT- NBRC offers neurological outpatient services at Civil Hospital, Gurgaon to patients from the city as well as neighbouring districts. Patients with epilepsy continue to come to NBRC for sophisticated investigations using the Magnetoencephalography (MEG) facility set up in collaboration with AIIMS, New Delhi. ‘DALI’ a tool in Indian languages for dyslexia assessment in children developed at NBRC remains in high demand and is being increasingly adopted countrywide.
NBRC scientists have developed specialized tools named BRAHMA and ANSH for integrating inputs from neuroimaging data and the clinical information to help in diagnosis of various brain diseases such as Alzheimer’s and Parkinson’s. The tool is standardized on a robust Indian brain template that is representative of the Indian population-specific brain anatomy.
Bharat Immunologicals & Biologicals Corporation Limited (BIBCOL), Bulandshahar, Uttar Pradesh
Hand sanitizer against COVID - 19
Zinc + Vitamin Tablets were developed and license has been obtained as an immunity booster tablets under FSSAI Act under food supplement category. The formulation consists of Zn, vitamin D3 and Vitamin C as active ingredients as per RDA recommendations. The formulation is being targeted as general immunity enhancement food supplement. The product is also being promoted through e-commerce.
*****
NB/KGS/(DBT inputs)
Salient Achievements 2020-2021
The Department of Biotechnology has made tremendous efforts in promoting Bioscience research, translational education and entrepreneurship. The Department has laid emphasis on the generation of biotech products, processes and technologies for enhanced efficiency, productivity and cost-effectiveness in the areas of agriculture, food and nutritional security; affordable health care and wellness; environmental safety; clean energy and biofuel, bio- manufacturing etc. Skill development programmes have been developed in close coordination with State Governments. The Department has contributed through its various programmes to the National Missions launched by the Prime Minister- Swasth Bharat, Swatch Bharat, Start-up India, Make in India and Digital India. The Department has also risen to the challenge for mitigation of COVID Pandemic by supporting vaccines, therapeutics, diagnostics, genomics, bioreporsitories and a National Platform (NBRIC) for achievement of Atmanirbharta.
Snap shot of achievements: The coordinated efforts of all the stakeholders resulted in supporting more than1642 projects, 2731Scientists and 5145 project fellows, more than 1 Lakh students were trained under Star college scheme, 40 Ramalingaswami Re-entry fellows secured regular appointment, 20,000 rural, SC,ST and Women beneficiaries were supported through Societal programme and more than 25,000 farmers supported thorough Biotech KISAN scheme.
UNATI Atal Jai Anusandhan Mission Programmes
UNATI Atal Jai Anusandhan Mission has been implemented by DBT with a major focus on improved agriculture, affordable healthcare, clean energy and cutting-edge frontier science as per details given below:

Ind-CEPI MISSION is an India centric collaborative mission of DBT aligned to the global initiatives of CEPI (Coalition of Epidemic Preparedness Innovations). DBT is supporting the implementation of the Ind-CEPIs mission “Epidemic preparedness through rapid vaccine development: Support of Indian vaccine development is aligned with the global initiative of the Coalition for Epidemic Preparedness Innovations (CEPI)”, at BIRAC, PSU of DBT.
Ind-CEPI Mission initiated the eCourse Series entitled “Strengthening Clinical Trial Research Capacity in Neighbouring Countries” primarily aimed towards skill development, capacity building and regional networking and coordination. A total of 4-Program 10 sessions series were conducted via online platform with total engagement of more than 750 participants from neighbouring countries like Afghanistan, Bangladesh, Bhutan, Maldives, Mauritius, Nepal and Srilanka.

DBTs response to COVID
Mission COVID Suraksha was announced with a provision of Rs. 900 Cr. to DBT for supporting development of a comprehensive ecosystem for enabling the development of a safe, efficacious and affordable vaccine for COVID-19. The milestone is to accelerate the development of at least 5-6 vaccine candidates and ensure that some of these are brought closer to licensure for consideration of regulatory authorities and for introduction in public health systems.

Highlights of major activities include:

COVID Vaccines:
MEA accorded 'in principle' approval for financial support for phase 3 trial of COVAXIN in Bangladesh by Bharat Biotech International ltd. (BBIL). Signing of a tripartite HoA with BBIL and ICMR, to finalize the modalities and issues related to the conduct of a COVAXIN based Phase III Efficacy trial in Bangladesh is under consideration. The revised budgetary requirement provided by BBIL has been shared with MEA. The semi-executed tripartite Heads of Agreement was shared with ICMR, to finalize the modalities and issues related to the conduct of a COVAXIN based Phase III Efficacy trial in Bangladesh is under consideration. The proposal for the conduct of clinical trials of indigenously developed inactivated vaccine (COVAXIN) by Bharat Biotech International Ltd.(BBIL), in Bangladesh, is under consideration by the Ministry of External Affairs (MEA). DBT-BIRAC is facilitating the same.
· CDSCO had undertaken an inspection of the laboratory infrastructure and the facilities, at NCCS, Pune and NIAB, Hyderabad, for assessing their functioning as Central Drug Laboratories for COVID-19 vaccine testing. A detailed project report is being prepared for consideration of MoH&FW for expansion of the facilities.
· A discussion of Secretary, DBT with Officials from Myanmar was held on 21st December, 2020 to explore opportunities for association with DBT and procurement and deployment of Indian COVID-19 vaccines in Myanmar.
· A discussion was held with the leadership of CEPI on 21st December 2020 to discuss the potential contribution of CEPI to the clinical development of the RBD based protein subunit vaccine candidate for COVID-19, which was positively reviewed in the third call for proposals given by CEPI for COVID-19 vaccine candidate development.
COVID Therapeutics:
DBT-AYUSH partnership
DCGI approved First Phytopharma drug
Immunoglobulin based therapeutics
Clinical trial for equine immunoglobulin therapy to start shortly
DBT-RCB has set an in vitro cell culture-based assay to test the antiviral activity of potential molecules against SARS-CoV2. Services have been widely utilized by the academia and industry. Cytotoxicity Testing and anti-viral testing was done on 679 and 313 samples respectively
COVID Diagnostics:
Covid Genomics:
Augmentation of self reliance and support to Start-ups:

COVID Start-up solutions:

COVID Biorepositories:
Sectoral Achievements:
Health:
Product Development
Shared Facilities
Skill Development
Technology Transfer
Vaccines
11 vaccine candidates for Flu, Cholera, Dengue, Pneumonia, and COVID, under different stages of development are being supported
National Facilities
Creation of GLP, GMP, GCLP facilities besides cell line repositories and facilities for medical device testing and prototyping. Establishment of 19 national facilities are ongoing, out of which 04 are already operational.
Trainings & Workshops
1406 candidates have been trained in the areas of clinical research, regulatory compliances, technology transfer, biopharmaceuticals and medical devices. 37.12% of the participants were female.
Tech Transfer Offices (TTO)
Five TTO’s have been established with a view to strengthen the technology transfer capacity of the country.
Biotherapeutic mAbs
About 12 mAbs which are not presently existing in Indian market and 03 clones for diseases like cancer, diabetes, psoriatic arthritis, wet macular degeneration and lung infections caused by RSV are being supported. Additionally, one antibody-drug conjugate (ADC), one novel biologic, one recombinant monoclonal and one bio-better are also being supported.
Translational Research Consortia (TRC)
The Mission has supported two consortia
(i.) TRC for Dengue: 3 clinical sites and 5 premier institutes across the country led by ICGEB, Delhi.
(ii.) TRC for Chikungunya (CHKV): 4 hospitals and 3 premier research institutes across the county, led by Manipal Academy of Higher Education.
Device & Diagnostics
Currently 17 devices and 13 diagnostics are being supported. Support is being given for development of products in the areas of hospital-use equipment, diagnostic imaging, implants, wound-care products etc. Many diagnostic devices and reagents used for diagnostic kits are also supported including Molecular diagnostics, ELISA, LFT, sample transport reagents etc.
Clinical Trial Networks (CTN)
CTN for hospital-based trials in patients for testing biologicals in different specialties of oncology, diabetology, rheumatology and ophthalmology. Currently, 05 consortia including 36 hospitals are being supported. Strengthening the capacity of vaccine clinical trials, in already existing Demographic Surveillance sites and establishing new sites is another major focus area. Ten (10) Field Sites have been prepared for the same.





Agriculture and Allied Areas:
Agriculture Biotechnology

A few crop wise major achievements are placed below:

Animal Biotechnology:
Process/Product/ Technology Developed:
Process/Product/Technology Transferred to User Agency/ Industry/ Stakeholders:
Process/Product/Technology Commercialized:
Aquaculture:
Energy, Bioresources, Environment and Forest Biotechnology:
Knowledge Generation:

DBT-HRD Salient Achievements 2020-21:
DBT-Skill Vigyan Programme:
DBT Star College Scheme
Infrastructure
· Initiation of 3rd Phase of the “Access to Structural Biology Facilities at ESRF, France”: The European Synchrotron Radiation Facility (ESRF), located in Grenoble, France, is the world's most intense X-ray source and a centre of excellence for fundamental and innovation-driven research in condensed and living matter science. More than 300 Ph.D students and 1.52 lakhs researchers have utilized the services till date and has led to publication of more than 500 high impact research articles.
Conference, Travel, Exhibition and Popular Lectures (CTEP)
Societal Programme
Biotech-KISAN has created a platform in each of 15 agro-climatic zones of the country, which aims to connect farmers and scientists to promote Agriculture Innovation and take the new interventions to the farmers and farms. Biotech-KISAN Hubs have been established at pilot-scale level in all agro-climatic zones. The programme has now been scaled up and expanded its activities covering 105 Aspirational Districts in the country. The programme will lead to link the farmers with the latest scientific innovations with a view to enhance agricultural production and increasing farmers’ income. The programme has introduced 200 interventions for the benefit of farming community and more than 25000 farmers have been benefitted so far.
International Cooperation:
NER Program
Personals Trained in NER
Publications
Patents filed/Granted
Technologies transferred
Bioformulations developed at CoE AAU Jorhat –NECAB include Talc based formulations (Bio-Time; Biozin-PTB; Bio-Sona; Biollium, Bio-Meta; Bio-Zium; Bio-veer; Biogreen; Biogreen-L) and Organic based (Biofor-Pf). MoU was signed for transferring Bio-input (Biopesticide production technology) Technology to the three companies: VRS Agritech, Guwahati, School of Livelihood & Rural Development (SLRD), Shillong, M/s Orgaman R&D Division, Jorhat.
Entrepreneurship Development
Biotechnology Industry Research Assistant Council (BIRAC)

`
DBT - Autonomous Institutions
16 Autonomous Institutions are functioning under the aegis of the Department of Biotechnology. These institutions are pursuing basic, discovery and translational research in line with the National missions in the areas of Agriculture Biotechnology, Animal Biotechnology and health, Medical Biotechnology, Clean energy and Bioresourse development, Secondary Agriculture etc. The institutions also have a mandate of human resource development and societal outreach. Some of the achievements during the year are given below:
DBT-National Institute of Immunology (DBT-NII) has developed Mycobacterium indicuspranii based leprosy vaccine and immunomodulator. This is being marketed by Cadila Pharmaceuticals, Ahmedabad as Immuvac. DBT-NII has published 85 research papers in reputed journals, was granted 5 patents and filed 11 patent applications. In order to protect intellectual property rights of the research endeavors, two trademarks, ASPAGNII and PANII have been filed for vaccine and biotechnological composition respectively. During this year, the Institute has also sealed a Technology Transfer Agreement with Vitane Biologicals.
DBT-Center of Innovative and Applied Bioprocessing (DBT-CIAB) has received the granted status of its 7 patents on various bioprocess based technologies/products developed by the institute. 5 new technologies were also developed in the areas of biomass valorization and bioprocess technology. R & D has established that low-methoxyl pectin stabilizes low-fat set yoghurt and improves their physicochemical properties, rheology, microstructure and sensory liking. Processes for removal of bitterness from kinnow pomace and kinnow pulp residue were also developed.
DBT-National Institute of Animal Biotechnology (DBT-NIAB), Hyderabad has developed a high-density SNP chip using NGS of 43 Indian breeds of cows. This chip is currently under validation using 50 samples of each breed to identify breed specific signatures. NIAB developed a kit for detection of subclinical and clinical mastitis and assessment of microbial quality of milk using magnetic nanoparticles, without the need of any high end equipment . The overall cost to perform one test is less than US $ 0.50/test.
DBT-Regional Centre for Biotechnology (DBT-RCB): RCB has been providing service to academia and industry for testing potential antivirals against SARS-CoV-2 in the cell culture model. So far, over 900 compounds or herbal preparations have been tested. More than 100 students are pursuing doctoral degree programs in Biotechnology, Bioinformatics, and Biostatistics in different RCB laboratories. 25 students are currently registered with RCB for integrated MSc-PhD degree program.
DBT-National Agri-Food Biotechnology Institute (DBT-NABI) has developed a wheat straw polysaccharide derived novel edible coating formulation to prevent the post-harvest shelf-life of perishable fruit crops such as Apple, Peach and Banana. In addition to that, antibody assisted graphene oxide coated gold nanoparticles for rapid bacterial detection and near infrared light enhanced antibacterial activity has been developed which detects food borne pathogens.
DBT- Rajiv Gandhi Centre for Biotechnology (DBT-RGCB): DBT- RGCB has established a nationwide investigator network for research on Head and Neck Cancer ( HNC) alongwith a HNC Bio-repository Utroside-B was identified as a potential anticancer agent against liver Cancer. This compound was licenced to Q-Biomed for further development in a joint initiative with RGCB and OMRF.
DBT-inStem: DBT- inStem was ranked in the top 50 among Indian institutions and 7th in the Life Sciences in India by Nature insex 2020 and has more than thirty publications to its credit since April 2020. InStem scientists have devised probes to enable understanding the many roles of cellular actin which is an important conserved protein from microorganisms to complex organisms and is an emerging drug-target in malaria, leishmaniasis amongst others. Under the aegis of the ADBS programme a recent study examined the consequences of adverse childhood experiences and published a study that has taken an impressive leap forward in modelling Autism – a major and growing public health challenge – in the culture dish. Together, these observations offer new potential therapeutic targets that can be assessed in pre-clinical platforms and eventually lead to more efficient translation to the clinic.
DBT- National Centre for Cell Science (DBT-NCCS): A GMP-Compliant National Repository for banking, safe deposit and supply of characterized mammalian cells for use in Bio-Pharma under BIRAC’s National Biopharma Mission was established in the institute. Promising proteins were identified that could serve as biomarkers and therapeutic targets for multiple myeloma, the second most prevalent blood cancer at the global level.
DBT- Institute of Bioresources and Sustainable Development (DBT-IBSD): Himalayan Bioresources Mission was initiated to connect the East and West, with major emphasis on the development of bio resources of Himalaya and their utilization for future development. In microbial resource programme, microbial repository deposits have increased to more than 72,000 cultures including bacteria, fungi, actinomycetes and yeasts.
DBT-Centre for DNA Fingerprinting and Diagnostics (DBT-CDFD) has provided a) High quality forensic DNA fingerprinting services in 33 cases to the various courts of law, law enforcement and investigative agencies of the country; b) Genetic diagnostic services, prenatal diagnosis, and clinical genetic counselling services to more than 500 people, c) Plant DNA fingerprinting services for 490 samples. 33 research papers have been published and 1 patent was filed.
DBT-National Institute of Plant Genome Research New Delhi: Through marker-assisted selection, large seed characteristic has been transferred into a commercially important desi chickpea variety, JG11 (ICCV 93954). The Improved JG1 variety has a 15-20% increase in yield over the parent. This variety is undergoing Advanced Varietal Trial-2 (AVT-2) at 16 different rainfed areas of India.
Translational Health Science and Technology Institute (THSTI), Faridabad established a dengue virus repository of 40 isolates that are being characterized for vaccine development, dengue structural E protein platform to support antibody-based therapeutic discovery and development and AG129 mouse breeding and colony to facilitate dengue research, Identified a novel HIV-1 Indian clade C native like trimer antigen that has demonstrated favourable immunogenicity in rabbit model, solved the crystal structure of vapB12 antitoxin and generated a hetero-octameric model of vapBC12 toxin-antitoxin complex etc. DBT-THSTI established the following national facilities to support vaccine development:
• The institute has published 28 papers, filed 8 patent applications, one copyright application, one trademark application,4 technology developed, 4 technologies transferred, and 1 technology has been commercialized during the year.
DBT- Institute of Life Sciences (DBT-ILS): The institute published 35 research publications during the year 2020. A novel target for regaining sensitivity towards Tamoxifen in case of breast cancer was identified. Human IRGM was shown as a master suppressor of interferon-signaling and targeting IRGM induces a broad antiviral response which can be further developed for anti-viral therapeutics. The Institute of Life Sciences has transferred a technology for production of curcumin sponge for wound healing for commercialization.
DBT-International Center for Genetic Engineering and Biotechnology (DBT-ICGEB): ICGEB scientists during the year were able to raise 4.92 million USD through competitive external project funding and also published 104 publications in peer-reviewed journals. Institute has filed one (1) national and three (3) international Patents and was granted one (1) Patent in 2020.
A link was established between Mtb infection, epigenetics and host immune response, which can be exploited to achieve therapeutic benefits. Using in silico and in vitro screening strategies, potent inhibitors of major cysteine proteases of Plasmodium falciparum, falcipain-2/3, were identified (Bioorg Med Chem. 2020. 28:115155.). Trehalose accumulation was achieved in transgenic rice plants which enabled these plants to tolerate multiple abiotic stresses such as salinity, drought and sodicity and show minimum yield penalty under stress conditions.
DBT- National Institute of Biomedical Genomics (DBT-NIBMG), Kalyani: The ICGC – India Project team of NIBMG participated in the Pan Cancer Analysis of Whole Genomes, a large scale international collaborative initiative of ICGC and TCGA which undertook integrative analysis of 2,658 whole-cancer genomes and their matching normal tissues across 38 tumour types from the Pan-Cancer Analysis of Whole Genomes (PCAWG) Consortium of the International Cancer Genome Consortium (ICGC) and The Cancer Genome Atlas (TCGA). The habit of using RAN/tobacco and GSTP1 AA-genotype together play a significant role in predisposition to oral cancer risk. progress. The institute has published 29 articles, has supported 30 PhD students and 17 iPhD students.
DBT-National Brain Research Institute (DBT-NBRC), Manesar: Japanese Encephalitis virus (JEV) infection was found to induce classical activation (M1) of microglia that drives the production of pro-inflammatory cytokines, while suppressing alternative activation (M2) that could serve to dampen the inflammatory response, thereby inducing inflammatory response, microglial activation, and neuronal apoptosis.Sleep loss, a pathological condition during aging and Alzheimer’s disease, led to impairment of translation at synapses associated with memory storage. DBT- NBRC offers neurological outpatient services at Civil Hospital, Gurgaon to patients from the city as well as neighbouring districts. Patients with epilepsy continue to come to NBRC for sophisticated investigations using the Magnetoencephalography (MEG) facility set up in collaboration with AIIMS, New Delhi. ‘DALI’ a tool in Indian languages for dyslexia assessment in children developed at NBRC remains in high demand and is being increasingly adopted countrywide.
NBRC scientists have developed specialized tools named BRAHMA and ANSH for integrating inputs from neuroimaging data and the clinical information to help in diagnosis of various brain diseases such as Alzheimer’s and Parkinson’s. The tool is standardized on a robust Indian brain template that is representative of the Indian population-specific brain anatomy.
Bharat Immunologicals & Biologicals Corporation Limited (BIBCOL), Bulandshahar, Uttar Pradesh
Hand sanitizer against COVID - 19
Zinc + Vitamin Tablets were developed and license has been obtained as an immunity booster tablets under FSSAI Act under food supplement category. The formulation consists of Zn, vitamin D3 and Vitamin C as active ingredients as per RDA recommendations. The formulation is being targeted as general immunity enhancement food supplement. The product is also being promoted through e-commerce.
*****
NB/KGS/(DBT inputs)